Mira Fertility System Offers Tech Breakthrough
Mira Fertility System Offers Tech Breakthrough
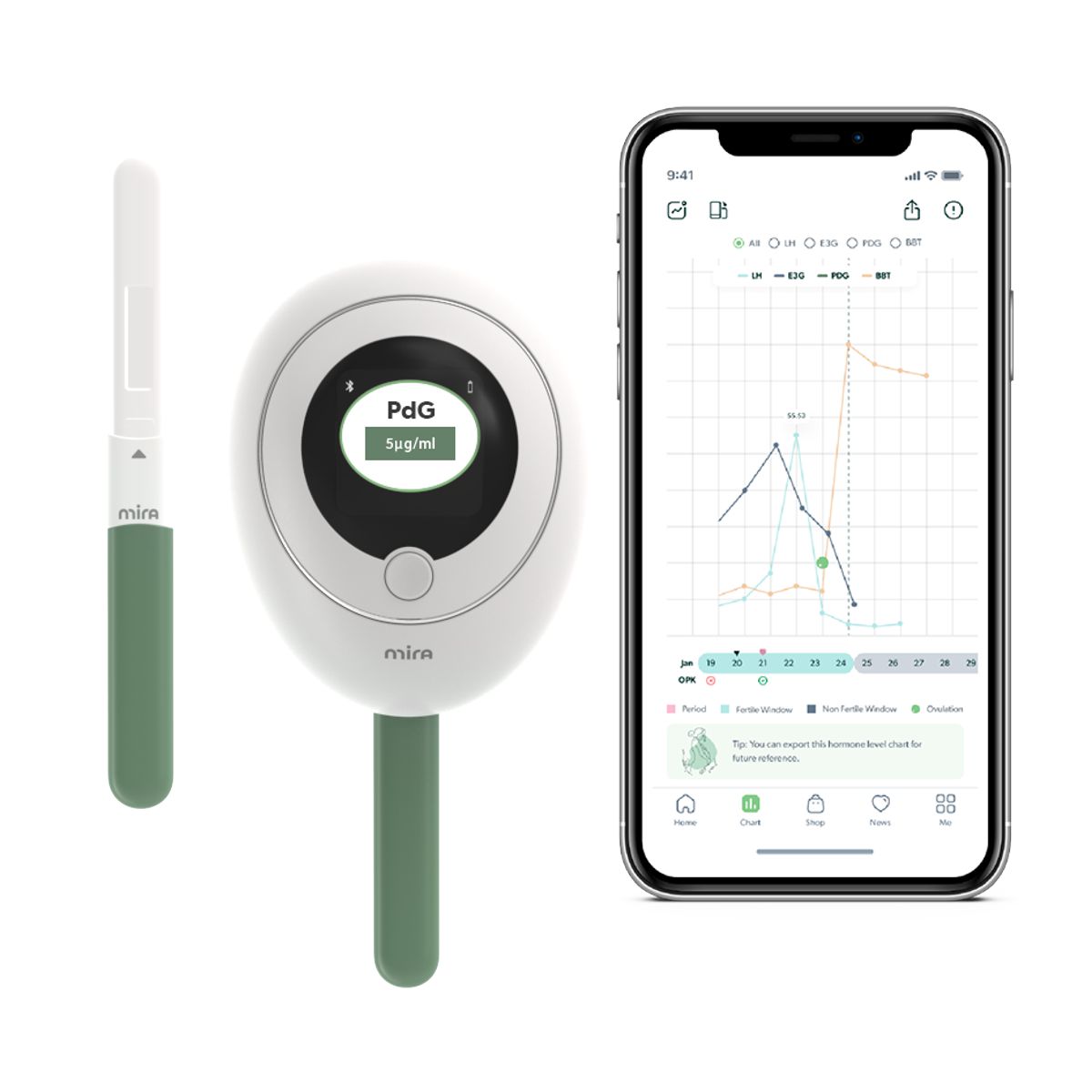
Quanovate Tech Inc. is the creator of Mira Fertility, the world's first fertility testing system to test, display, and track actual hormone concentrations. By leveraging the latest developments in digital health, machine learning, and AI based analysis, executives say the company is advancing home health by making reproductive health tracking simple and more accurate.
In October 2021, Mira's Progesterone (PdG) Confirm Wand received a greenlight from the FDA, which allowed its entry into the U.S. market. Mira provides a safe, non-invasive, and fast alternative for hormone testing from the comfort of the home.
"The Mira system is considered as a Class I medical device, which means that it has minimal to no potential risk to the user," says Sylvia Kang, CEO and Co-founder of Mira. "Getting reviewed and passing the FDA's rigorous review proves that it's safe and effective."
To be FDA-registered, all medical devices need to submit requirements such as clinical trials and performance research to the FDA. The PdG Confirm Wand is the newest addition to the company's existing line of hormone test wands. Prior to its launch, Mira had two hormone wands, Mira Fertility (luteinizing hormone or LH) and Mira Fertility Plus (luteinizing hormone and estrogen).
"With the launch of PdG wands, we now have a complete system that gives individuals a holistic view of their cycle and obstetric health," notes Kang. "For existing and future Mira users, obtaining the FDA mark for Confirm wands means that the device is now approved as an accurate tool to confirm ovulation."
Mira Confirm Wand is compatible with the Mira Analyzer, a high-precision, fluorescent-based testing device that uses patented machine learning to track a person's unique and ever-changing hormone patterns measured over time. This further sets the AI-powered Mira System apart from over-the-counter ovulation strips. On the Mira App, these patterns are displayed in easy-to-understand visual and numerical data along with personalized insights on a user's reproductive health.
Mira's Confirm Wand can be used as a standalone or in combination with other Mira wands. A 2021 study found that, when wands were used in combination, Mira shows 28.6% higher accuracy than its major competitor and is able to detect whether ovulation has occurred with 99% accuracy, correlating with results of standard lab tests, according to company officials.
"Mira's ability to accurately measure and track progesterone levels at home is a technological breakthrough helping to support women on their journey to conception," says Apurva Shah, a board-certified Ob-Gyn and member of the Mira Medical Advisory Board. "Until now, there was a lack of clarity on the progress of the cycle after the predicted fertility window detected with LH kits. PdG wands provide the much needed clarification by confirming ovulation and sustained luteinization until the first detection of pregnancy."
Mira is the first at-home solution to offer a personalized and lab-quality tracking system for monitoring women's hormones. It uses patented AI algorithms to accurately measure levels of major female hormones to provide a holistic view of reproductive health. Mira is FDA-approved, clinically approved, and recommended by medical specialists, according to Quanovate Tech executives.
Quanovate opened its headquarters at Hacienda in March 2018. The company was founded in late 2015 by a group of scientists, engineers, medical specialists, and business executives to provide healthcare products for more personal, accurate, and reliable home healthcare.
Quanovate's first healthcare product for at-home testing, Mira Fertility, was released in 2018.
For more information on Quanovate Tech Inc. and Mira, please visit www.miracare.com.




